 This week, IQPC released the final report of a very interesting survey they conducted in April through June this year. The results are fresh and they paint a picture with good news, and not so good news about the readiness of pharmaceutical manufacturers facing serialization deadlines in the United States, the European Union and elsewhere. The survey focused on serialization planning, implementation progress, traceability in operation and benefits beyond compliance. You can download the full report here, but let’s take a look at the responses to just one of the questions they asked.
This week, IQPC released the final report of a very interesting survey they conducted in April through June this year. The results are fresh and they paint a picture with good news, and not so good news about the readiness of pharmaceutical manufacturers facing serialization deadlines in the United States, the European Union and elsewhere. The survey focused on serialization planning, implementation progress, traceability in operation and benefits beyond compliance. You can download the full report here, but let’s take a look at the responses to just one of the questions they asked.
HOW LONG?
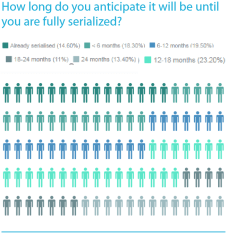
They asked, “How long do you anticipate it will be until you are fully serialized?” The question was asked of companies who market drugs all around the world, but mainly in the U.S. and E.U., so it is surprising that one quarter of the respondents said either “18-24 months”, or “24 months”. It means those companies are admitting that they are going to miss the deadlines in both the U.S. and the E.U.
Because of the recent one year delay in enforcement of the serialization requirement by the U.S. FDA (see “FDA Delays Enforcement of DSCSA November Deadline: What It Means”), the deadlines of the two markets are now less than 10 weeks apart: November 27, 2018 in the U.S. and February 9, 2019 in the E.U. I have several European industry friends who insist the Falsified Medicines Directive Delegated Regulation deadline will not be pushed out. I’m not sure I believe them—even before seeing the results of this survey—but now I believe them even less. A regulator cannot risk one quarter of the manufacturers supplying drugs to their citizens being restricted from doing so, just because they are still six months away from being ready to serialize. The U.S. FDA realized that. The European Medicines Agency (EMA) will figure it out too. But that raises another question.
HOW SOON?
if EMA delays the start of their serialization requirement, how soon should they make that announcement? There is an argument that they should wait until the last minute. That may seem logical in theory, but in practice I don’t think that’s going to work so well—especially with the FMD/DR. Remember, the FMD/DR is a “big bang”. That is, not only does the serialization requirement start on February 9, 2019, but so does the pharmacy verification requirement, for serialized drugs. In fact, all of the FMD/DR requirements start on that date. That’s pretty hard to coordinate (see “Insufficient Transitional Measures Doom The FMD-EUDA” and “More Concerns With The FMD/EUDR Big Bang Start”).
In the U.S., we have a series of many deadlines across a 10 year period to get from the start to the final, full traceability requirements. Delaying the serialization requirements here was complicated enough (see “FDA Delays Enforcement of DSCSA November Deadline: What It Means”, “DSCSA and RxTrace: The Song Remains The Same” and “DSCSA Cascading Delays”), for a delay to work in the E.U., I think it’s going to take more than the five months the FDA gave the industry here.
So the question remains, in the face of surveys like this one from IQPC, how soon will they let us know?
 Fortunately there is an event coming up that may help document the real current state of preparations for both the U.S. and the E.U. requirements. It’s the Pharmaceutical Traceability Forum | Interactive, held this year in Washington DC on December 7th and 8th. That’s the absolute perfect location and timing, because the second FDA DSCSA Public Meeting will be held in the DC area on December 5th and 6th (see “FDA Announces New DSCSA Pilot Program and Public Meeting Series“).
Fortunately there is an event coming up that may help document the real current state of preparations for both the U.S. and the E.U. requirements. It’s the Pharmaceutical Traceability Forum | Interactive, held this year in Washington DC on December 7th and 8th. That’s the absolute perfect location and timing, because the second FDA DSCSA Public Meeting will be held in the DC area on December 5th and 6th (see “FDA Announces New DSCSA Pilot Program and Public Meeting Series“).
Those of you who are attending the FDA meeting can save on travel by staying an extra two days and learn what is going on in other companies as they grapple with the same deadlines around the world that you are. The speaker lineup is stellar, a veritable who’s who of pharma serialization veterans. They should be full of fresh valuable information after having just attended the FDA Public Meeting. So you can benefit, even if you registered too late to get into the FDA meeting. This is looking like the best event of the fall season covering global pharma serialization and traceability. Don’t miss it.
Dirk.
