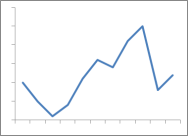
Last week I announced the availability of the 2015 RxTrace U.S. Pharma Traceability Survey Results that are sponsored by Frequentz. You should download a free copy of the report here. This week I want to look at another interesting finding taken directly from the report. It shows that progress is being made by drug manufacturers, repackagers and CMO/CPOs toward meeting the 2017 (2018 for repackagers) deadline for adding serial numbers to the drug packages they produce for the U.S. market. That requirement comes from the Drug Supply Chain Security Act (DSCSA) enacted in November of 2013.
Last week I announced the availability of the 2015 RxTrace U.S. Pharma Traceability Survey Results that are sponsored by Frequentz. You should download a free copy of the report here. This week I want to look at another interesting finding taken directly from the report. It shows that progress is being made by drug manufacturers, repackagers and CMO/CPOs toward meeting the 2017 (2018 for repackagers) deadline for adding serial numbers to the drug packages they produce for the U.S. market. That requirement comes from the Drug Supply Chain Security Act (DSCSA) enacted in November of 2013.
One of the many questions we asked Continue reading Progress Toward Serialization!?